On behalf of the Microbiome First Virtual Summit planning committee, we warmly welcome you to this years event taking place at a Web browser near you. Whether you're logged in at your residence, the office or in the park somewhere, we feel privileged that you could join us.
Today, May 17, 2022 marks Day 1 of the 3 day event. We hope that you will enjoy this very special event.
Note: If you have a Twitter account you can follow along or ask questions at @Microbiomefirst or #MicrobiomeFirstSummit
* if you want to watch videos in full screen mouse over and click the "full screen" icon on the bottom right corner of the speakers video.
* You can also play "subtitles" in English by mousing over the "CC" icon also in the right bottom corner of the speakers video.
Our Keynote speaker, "Big Picture View of Our Tiny Microbes"
by RODNEY DIETERT, PHD
Cornell University Professor Emeritus
Ithaca, NY, USA
Author of The Human Superorganism.
World Asthma Foundation
Session: Impact and Burden of Non
Communicable Disease NCD.
MARIE-CLAIRE ARRIETA, PHD
Associate Professor, departments of Physiology, Pharmacology, and Pediatrics, University of Calgary
Calgary AB, CANADA
Session: "The early-life mycobiome in immune and metabolic development"
JUSTIN SONNENBURG, PHD
Senior research scientist and Associate Professor in the Department of Microbiology and Immunology at the Stanford University School of Medicine.
Palo Alto, CA, USA
Session: "Gut-microbiota-targeted diets modulate human immune status"
KATRINE L. WHITESON, PHD
Assistant Professor, Molecular Biology and Biochemistry School of Biological Sciences
Associate Director, UCI Microbiome Initiative
Irvine, CA, USA
Session: "High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids"
LISA AZIZ-ZADEH, PHD
Cognitive neuroscientist; Expert in brain imaging, autism, body cognition
Associate Professor in the USC Chan Division of Occupational Science and Occupational Therapy
Los Angeles, CA, USA
Session: "Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder"
LIEKE VAN DEN ELSEN, PHD
Research Fellow, The University of Western Australia, Australia
Honorary Research Associate, Telethon Kids Institute.
Perth, WA, AUSTRALIA
Session: "Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention"
A quick wrapup of what we learned today, from the speakers above.
On behalf of the Microbiome First Virtual Summit planning committee, we warmly welcome you to this years event taking place at a Web browser near you. Whether you're logged in at your residence, the office or in the park somewhere, we feel privileged that you could join us.
Today, May 18, 2022 marks Day 2 of the 3 day event. We hope that you will enjoy this very special event.
Note: If you have a Twitter account you can follow along or ask questions at @Microbiomefirst or #MicrobiomeFirstSummit
* if you want to watch videos in full screen mouse over and click the "full screen" icon on the bottom right corner of the speakers video.
* You can also play "subtitles" in English by mousing over the "CC" icon also in the right bottom corner of the speakers video.
BENOIT CHASSAING, PHD
Principal Investigator, Chassaing Lab
Associate professor, French National Institute of Health and Medical Research.
Paris, FRANCE
Session: "Ubiquitous food additive and microbiota and intestinal environment"
PATRICIA MACCHIAVERNI, PHD
Clinical and translational researcher
Research Fellow, The University of Western Australia
Perth, WA, AUSTRALIA
Honorary Research Associate, Telethon Kids Institute.
Session: "House Dust Mite Shedding in Human Milk: a Neglected Cause of Allergy Susceptibility?"
CLAUDIA S. MILLER, MD, MS
Emeritus Professor, Allergy/Immunology and Environmental Health University of Texas San Antonio, TX, USA
Session: "Toxicant-Induced Lost of Tolerance for Chemicals, Foods and Drugs: a Global Phenomenon"
JAEYUN SUNG, PHD
Assistant Professor, Microbiome Program, Center for Individualized Medicine, Mayo Clinic.
Rochester, MN, USA
Session: "A predictive index for health status using species-level gut microbiome profiling"
MARTIN KRIEGEL, MD, PHD
Chief of Rheumatology and Clinical Immunology at University Hospital of Münster
GERMANY
Associate Professor Adjunct of Immunobiology at Yale School of Medicine.
Session: "Dietary Resistant Starch Effects on Gut Pathobiont Translocation and Systemic Autoimmunity"
EMMA HAMILTON-WILLIAMS, PHD
Associate Professor
Principal Research Fellow
The University of Queensland Diamantina Institute
Faculty of Medicine
The University of Queensland
Translational Research Institute
Woolloongabba, QLD, AUSTRALIA
Session: "Metabolite-based Dietary Supplementation in Human Type 1 Diabetes is associated with Microbiota and Immune modulation"
https://vimeo.com/709642859/b910429a39
SEI WON LEE, MD, PHD
Associate Professor
College of Medicine, University of Ulsan
Department of Pulmonary and Critical Care, Asan Medical Center
Seoul, KOREA
Session: "The Therapeutic Application of Gut-Lung Axis in Chronic Respiratory Disease"
EMERAN A MAYER, MD
Gastroenterologist, Neuroscientist, Distinguished Research Professor
Department of Medicine, UCLA David Geffen School of Medicine
Executive Director, G. Oppenheimer Center for Neurobiology of Stress and Resilience at UCLA
Founding Director, UCLA Brain Gut Microbiome Center.
Los Angeles, CA, USA
Session: "The Gut–Brain Axis and the Microbiome: Mechanisms and Clinical Implications"
Day 2 Wrap-up
On behalf of the Microbiome First Virtual Summit planning committee, we warmly welcome you to this years event taking place at a Web browser near you. Whether you're logged in at your residence, the office or in the park somewhere, we feel privileged that you could join us.
Today, May 19, 2022 marks Day 3 of the 3 day event. We hope that you will enjoy this very special event.
Note: If you have a Twitter account you can follow along or ask questions at @Microbiomefirst or #MicrobiomeFirstSummit
* if you want to watch videos in full screen mouse over and click the "full screen" icon on the bottom right corner of the speakers video.
* You can also play "subtitles" in English by mousing over the "CC" icon also in the right bottom corner of the speakers video.
Paul Turner, PhD
SESSION: "New Yale Center to Advance Phage Research,
Understanding, Treatments, Training, Education"
This session is Dedicated to the Memory of Mallory Smith "Salt in My Soul: An Unfinished Life Book" Mallory's "legacy lives on through her writing and phage therapy" according to Mallory's mother Diane Shader Smith who advocates on behalf phage education and treatment.
Andres Cubillos-Ruiz,PhD
SESSION: "Protecting the gut microbiota from antibiotics with engineered live biotherapeutics"
TONI HARMAN PRINCIPAL
Microbiome Courses Session: "Educating Parents About 'Seeding And Feeding' A Baby's Microbiome"
Wrapup Day 3
This is Alan Gray with a wrapup review of the third day of the Microbiome First Summit underwritten by the World Asthma Foundation.
Thank you for participating in the Summit.
Today, in the Current Clinical Trials and Future Therapies Tracks
We heard from Yale University’s Dr Paul Turner, who presented his session on Phage Research.
We learned about:
The possibility of phage therapy combating antibiotic resistant bacteria, the history of phage therapy research.
A comparison of phage therapy and chemical antibiotics, and potential synergies when they are used in combination.
Dr ANDRES CUBILLOS-RUIZ, a scientist with the Wyss Institute of Harvard University and Institute of Medical Engineering and Science at MIT spoke about "Protecting the Gut Microbiota from Antibiotics by using Engineered Live Biotherapeutics"
We learned that :
Antibiotic-induced alterations in the gut microbiota are implicated in a wide range of metabolic and inflammatory diseases, as well as with the emergence of antimicrobial resistance and increased risk to secondary infections.
β-lactams are the most widely used antibiotics and their broad-spectrum activity is known to cause major disruptions to commensal bacteria in the gut.
They Used a mouse model of ampicillin treatment, to demonstrate that oral supplementation with their engineered live biotherapeutic product (eLBP) minimizes dysbiosis in the gut without affecting the ampicillin concentration in the serum.
From Toni Harman we heard a practical application of microbiome research. We learned that informing parents about the critical microscopic events that take place during a normal birth and through breastfeeding can empower their choices before, during and after birth. Parents can take positive steps to nurture and protect their own gut microbiome, and thereby their child’s microbiome.
We hope you enjoyed the presentations on day 3, and that you’ll help us share this information with your friends, colleagues, students, and others who may benefit from this information.
This was the final day in the inaugural Microbiome First Summit underwritten by the World Asthma Foundation, and we expect there to be many more as microbiome research continues to reveal things we didn’t understand about our own bodies before.
The World Asthma Foundation is grateful to our Keynote speaker, Dr Rodney Dietert for his time in helping guide us over the past two years. We thank all of the high-calibre speakers who presented at this Microbiome First Summit over the past three days for the huge effort they put into their presentations, and for passing on the amazing information they learned through their research.
We also thank everyone who registered to participate in the summit, researchers, Non Communicable Disease communities, students and people who suffer from these diseases and companies that help bring new technologies and new products to market to improve the lives of people who suffer. We hope you all learned some things you didn’t know before, we hope researchers, students and entrepreneurs will be inspired to great things.
The two big things we hoped to do, when we thought about creating this Summit, was to first find ways to improve people’s lives, addressing the actual cause of their suffering and not just the symptoms, and second, to reduce the cost of making people well. This idea was advanced through our initial research, and came when we interviewed Dr. Dietert. The phrase “Microbiome First” came from a discussion with him, as did the idea of Sustainable Healthcare. This interview was part of our Defeating Asthma series, in which we interviewed Dr Martin Blaser, Dr Justin Sonnenburg, Dr Paul Bollyky, Dr Marie-Claire Arrieta and Dr. Nikolaos Papadopoulos.
Future
Please look for our next messages, where we plan to talk about the future for getting to practical help for those with non-communicable diseases. Later we will announce our next Summit.
Please follow @MicrobiomeFirst and @AsthmaFacts on twitter. We would love it if you would communicate with us about any ideas or suggestions you may have and of course participate in the conversation on twitter. Let's all collaborate and improve lives.
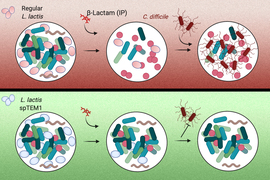
Engineered bacteria could help protect “good” gut microbes from antibiotics says researchers at Massachusetts Institute of Technology (MIT)
According to MIT news reports, researchers have engineered a strain of bacteria, noted as L. lactis spTEM1 in the image, that can help protect the natural flora of the human digestive tract from antibiotics and prevent opportunistic infections such as C. difficile from developing a Microbes that safely break down antibiotics could prevent opportunistic infections and reduce the spread of antibiotic resistance.
Credits:Image: Courtesy of the researchers, edited by MIT News
Why Microbiome Science Matters:
MIT researchers engineered a strain of bacteria, noted as L. lactis spTEM1 in the image, that can help protect the natural flora of the human digestive tract from antibiotics and prevent opportunistic infections such as C. difficile from developing.
Content and Image: Courtesy of the researchers, edited by MIT News
Antibiotics are life-saving drugs, but they can also harm the beneficial microbes that live in the human gut. Following antibiotic treatment, some patients are at risk of developing inflammation or opportunistic infections such as Clostridiodes difficile. Indiscriminate use of antibiotics on gut microbes can also contribute to the spread of resistance to the drugs.
In an effort to reduce those risks, MIT engineers have developed a new way to help protect the natural flora of the human digestive tract. They took a strain of bacteria that is safe for human consumption and engineered it to safely produce an enzyme that breaks down a class of antibiotics called beta-lactams. These include ampicillin, amoxicillin, and other commonly used drugs.
When this “living biotherapeutic” is given along with antibiotics, it protects the microbiota in the gut but allows the levels of antibiotics circulating in the bloodstream to remain high, the researchers found in a study of mice.
“This work shows that synthetic biology can be harnessed to create a new class of engineered therapeutics for reducing the adverse effects of antibiotics,” says James Collins, the Termeer Professor of Medical Engineering and Science in MIT’s Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering, and the senior author of the new study.
Andres Cubillos-Ruiz PhD ’15, a research scientist at IMES and the Wyss Institute for Biologically Inspired Engineering at Harvard University, is the lead author of the paper, which appears today in Nature Biomedical Engineering. Other authors include MIT graduate students Miguel Alcantar and Pablo Cardenas, Wyss Institute staff scientist Nina Donghia, and Broad Institute research scientist Julian Avila-Pacheco.
Protecting the gut
Over the past two decades, research has revealed that the microbes in the human gut play important roles in not only metabolism but also immune function and nervous system function.
“Throughout your life, these gut microbes assemble into a highly diverse community that accomplishes important functions in your body,” Cubillos-Ruiz says. “The problem comes when interventions such as medications or particular kinds of diets affect the composition of the microbiota and create an altered state, called dysbiosis. Some microbial groups disappear, and the metabolic activity of others increases. This unbalance can lead to various health issues.”
One major complication that can occur is infection of C. difficile, a microbe that commonly lives in the gut but doesn’t usually cause harm. When antibiotics kill off the strains that compete with C. difficile, however, these bacteria can take over and cause diarrhea and colitis. C. difficile infects about 500,000 people every year in the United States, and causes around 15,000 deaths.
Doctors sometimes prescribe probiotics (mixtures of beneficial bacteria) to people taking antibiotics, but those probiotics are usually also susceptible to antibiotics, and they don’t fully replicate the native microbiota found in the gut.
“Standard probiotics cannot compare to the diversity that the native microbes have,” Cubillos-Ruiz says. “They cannot accomplish the same functions as the native microbes that you have nurtured throughout your life.”
To protect the microbiota from antibiotics, the researchers decided to use modified bacteria. They engineered a strain of bacteria called Lactococcus lactis, which is normally used in cheese production, to deliver an enzyme that breaks down beta-lactam antibiotics. These drugs make up about 60 percent of the antibiotics prescribed in the United States.
When these bacteria are delivered orally, they transiently populate the intestines, where they secrete the enzyme, which is called beta-lactamase. This enzyme then breaks down antibiotics that reach the intestinal tract. When antibiotics are given orally, the drugs enter the bloodstream primarily from the stomach, so the drugs can still circulate in the body at high levels. This approach could also be used along with antibiotics that are injected, which also end up reaching the intestine. After their job is finished, the engineered bacteria are excreted through the digestive tract.
Using engineered bacteria that degrade antibiotics poses unique safety requirements: Beta-lactamase enzymes confer antibiotic resistance to harboring cells and their genes can readily spread between different bacteria. To address this, the researchers used a synthetic biology approach to recode the way the bacterium synthetizes the enzyme. They broke up the gene for beta-lactamase into two pieces, each of which encodes a fragment of the enzyme. These gene segments are located on different pieces of DNA, making it very unlikely that both gene segments would be transferred to another bacterial cell.
These beta-lactamase fragments are exported outside the cell where they reassemble, restoring the enzymatic function. Since the beta-lactamase is now free to diffuse in the surrounding environment, its activity becomes a “public good” for the gut bacterial communities. This prevents the engineered cells from gaining an advantage over the native gut microbes.
“Our biocontainment strategy enables the delivery of antibiotic-degrading enzymes to the gut without the risk of horizontal gene transfer to other bacteria or the acquisition of an added competitive advantage by the live biotherapeutic,” Cubillos-Ruiz says.
Maintaining microbial diversity
To test their approach, the researchers gave the mice two oral doses of the engineered bacteria for every injection of ampicillin. The engineered bacteria made their way to the intestine and began releasing beta-lactamase. In those mice, the researchers found that the amount of ampicillin circulating the bloodstream was as high as that in mice who did not receive the engineered bacteria.
In the gut, mice that received engineered bacteria maintained a much higher level of microbial diversity compared to mice that received only antibiotics. In those mice, microbial diversity levels dropped dramatically after they received ampicillin. Furthermore, none of the mice that received the engineered bacteria developed opportunistic C. difficile infections, while all of the mice who received only antibiotics showed high levels of C. difficile in the gut.
“This is a strong demonstration that this approach can protect the gut microbiota, while preserving the efficacy of the antibiotic, as you're not modifying the levels in the bloodstream,” Cubillos-Ruiz says.
The researchers also found that eliminating the evolutionary pressure of antibiotic treatment made it much less likely for the microbes of the gut to develop antibiotic resistance after treatment. In contrast, they did find many genes for antibiotic resistance in the microbes that survived in mice who received antibiotics but not the engineered bacteria. Those genes can be passed to harmful bacteria, worsening the problem of antibiotic resistance.
The researchers now plan to begin developing a version of the treatment that could be tested in people at high risk of developing acute diseases that stem from antibiotic-induced gut dysbiosis, and they hope that eventually, it could be used to protect anyone who needs to take antibiotics for infections outside the gut.
“If the antibiotic action is not needed in the gut, then you need to protect the microbiota. This is similar to when you get an X-ray, you wear a lead apron to protect the rest of your body from the ionizing radiation,” Cubillos-Ruiz says. “No previous intervention could offer this level of protection. With our new technology we can make antibiotics safer by preserving beneficial gut microbes and by reducing the chances of emergence of new antibiotic resistant variants.”